Test will be available to horse owners from September 2019
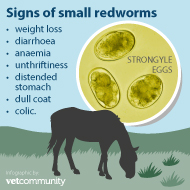
A new blood test to help diagnose small redworms in horses, including encysted larvae, has been developed by scientists at the Moredun Research Institute (MRI).
The test will be available to horse owners from September 2019 following extensive research and will be launched by commercial partner, Austin Davis Biologics (ADB).
Professor Jacqui Matthews, who led the Matthews research group at MRI, said: “It is great to see the commercialisation of this much-needed test to support sustainable worm control in horses.
“The test fills an important gap in our diagnostic toolbox and will enable horse owners to work with their veterinarians in targeting anthelmintic treatments against cyathostomin infections and hence help protect these important medicines for the future."
MRI business development manager Rhona Macdonald added: “We are delighted that the research has led to the development of a new blood test to help diagnose small redworms in horses and that the test is now available through Austin Davis Biologics.”
The new service provision marks the first phase in developing the blood test for commercialisation.
Dr Corrine Austin of ADB said: “We are thrilled to be making this test available to horse owners after extensive research has been conducted to achieve high accuracy. ADB are now developing laboratory ELISA [enzyme-linked immunosorbent assay] kits to enable independent veterinary laboratories to conduct blood testing.
“These kits are expected to reach market during 2020. Research into the saliva-based test is ongoing and is expected to be commercialised several years from now.”
Veterinary practices interested in the diagnostic test service can register their interest at info@austindavis.co.uk



 The latest
The latest